Biodiesel production (DL BIO-10)



PL-239848
The biodiesel is a liquid biofuel obtained by chemical processes from vegetable oils or animal fats and it is an alcohol that can be used in diesel engines, alone or blended with diesel oil. ASTM International (originally known as the American Society for Testing and Materials) defines the biodiesel as a mixture of long-chain mono alkyl esters from fatty acids obtained from renewable resources, to be used in diesel engines. The chemical reaction that converts a vegetable oil or animal fat to biodiesel is called "transesterification." This is a long name for a simple process of combining a chemical compound called an "ester" and an alcohol to make another ester and another alcohol. Oils and fats are included in the ester family. When they react with methanol or ethanol, they make methyl or ethyl esters and a new alcohol called glycerol or, more commonly, glycerin.
List of Experiments
Biodiesel synthesis and determination of the density of the product
Measurement of the biodiesel properties.
Measurement of combustion pollution through the soot produced during:
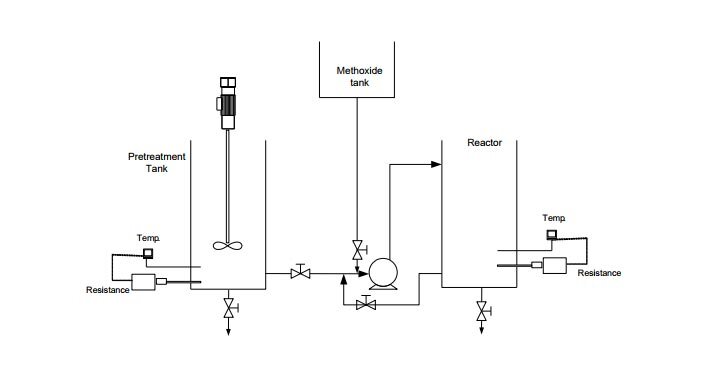
The tank is provided with flanged caps for easy dismantling and cleaning. It is used to perform the pretreatment of the oil, by means of a stirrer, a heating resistor and a temperature sensor with digital controller to maintain the desired value.
This reactor is a stainless steel tank of 200 mm. diameter and 350 mm. height for a volume of about 10 liters. This tank is also provided with flanged caps for easy dismantling and cleaning
By means of the same metering pump the methoxide, coming from a tank of 5 liters. made of plastic material, is added to the reactor.
The activation switches of the various elements (Resistances, Stirrer, Pump) are located on the front. There is also a general on-off switch and an emergency stop button.
The power supply is single-phase with neutral and earthing. Complete with:
Biodiesel production
The process to make biodiesel involves a chemical reaction. This means that the biodiesel industry is a chemical industry. Those involved in making biodiesel must have a good understanding of the underlying chemistry to ensure they are making quality fuel in a safe manner. Biodiesel is an alternative fuel for diesel engines. It is produced by chemically reacting a vegetable oil or animal fat with an alcohol such as methanol or ethanol. The vegetable oils and animal fats used to make biodiesel can come from virtually any source. All these products consist of chemicals called triglycerides; so, biodiesel can be made from soybean oil, canola oil, beef tallow, and pork lard, and even from such exotic oils as walnut oil or avocado oil. Even used cooking oil or waste oil can be used to make biodiesel. However, these oils present special challenges for the biodiesel production because they contain contaminants such as water, meat scraps, and breading that must be filtered out before the oil is converted to biodiesel. Methanol is the most common alcohol used for making biodiesel.
Description of the process
Oil and glycerin are inserted in the pretreatment tank and heated to 50°C, continuously stirring. The mixture is then settled and decanted to remove any impurities from the oil. The oil is then heated to 70-90 °C until the bubbles disappear. Then, by means of the pump, the oil is sent to the reactor and heated, while keeping the temperature between 50 and 55 °C. Methanol and sodium are mixed in the methoxide tank. The recirculation of the oil starts with the pump and is continued, while 75% of prepared methoxide is added in 1.5 hours. Let stand and decant. The recirculation of the oil starts again, while 20% of the methoxide is added in 1.5 hours. Let stand and decant. The recirculation of the oil starts again, while the remaining 5% of the methoxide is added in 1.5 hours. Let stand and decant. In this way biodiesel is obtained. The next step is to wash and purify it.
Necessary accessories to be provided locally for the correct functioning of the process:
What is this?
These percentage scores are an average of 0 user reviews. To get more into detail, see each review and comments as per below
If you have used this product, support the community by submitting your review



